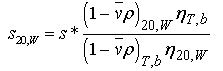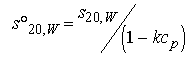Difference between revisions of "Converting s to s-20-w"
Bill Bashir (talk | contribs) (Created page with "The sedimentation coefficient provides a rigorous hydrodynamic descriptor of a molecule. However, to be most useful in this regard, and prior to further interpretation, s* must ...") |
(No difference)
|
Revision as of 14:52, 11 August 2011
The sedimentation coefficient provides a rigorous hydrodynamic descriptor of a molecule. However, to be most useful in this regard, and prior to further interpretation, s* must be standardized and corrected for concentration effects on s*. By convention, s* which has been standardized to conditions corresponding to pure water at 20 C and extrapolated to zero protein concentration is designated s20w. Because of the solvent-independent nature of s20w, it describes quantitatively the fundamental hydrodynamic properties of the protein and it is this value which is most useful in comparing the sedimentation behavior of different proteins. Moreover Sednterp must use s20w in calculating the limits of hydration and estimating the asymmetry of a macromolecule. Finally, comparisons of s20w determined for a molecule in solvents of differing composition can yield unique information concerning changes in macromolecular interactions, shapes, sizes and hydration. The corrections account for the solvent density ρ and viscosity η and the protein's partial specific volume vbar; ρ and vbar affect s* by their presence in the term (1-vbar*ρ) (eqn. 3) and η affects s* inversely through f.
Standardizing s*: s* is corrected to standard conditions of water at 20 oC using equation 19:
Equation 19:
where the subscripts refer to the experimental temperature (T, 20=20 C) and buffer conditions (b=buffer, w=water), respectively. Note that vbar in the numerator refers to the value at 20 oC, whereas vbar in the denominator should be corrected for temperature and should be corrected for specific ion effects whenever possible. (Ref. 6) The values for water at 20 C are p20w = 0.98234 g/ml and η20w =1.002 cp.
The concentration-dependence of s*: Prior to further analysis, s20w should be determined for a number of samples at different initial protein concentrations. A graph then should be constructed to extrapolate s20w to zero protein concentration. The nomenclature for a standardized, concentration-corrected (i.e. lim C->0) is s20w. In cases where well resolved boundaries are being examined, such corrections should be made for each component individually, if at all possible. The determination of s20w at various solute concentrations may reveal either increases or decreases in s with increasing solute concentration. A decrease in with increasing c is expected on hydrodynamic grounds. (Ref. 21 22) A rather weak dependence (about a 1% change in s per mg/ml) is observed for spherical molecules, whereas asymmetric molecules will exhibit a much stronger concentration dependence. (Ref. 23)
Increases in s20w with increasing solute concentration or the appearance and growth of faster-sedimenting boundaries at increased solute concentrations is diagnostic for mass-action association. The full analysis of sedimentation velocity data from interacting systems is reviewed in Analytical Ultracentrifugation in Biochemistry and Polymer Science and elsewhere. (Ref. 17 24)
In certain cases, the effect of protein concentration on sobs can be taken into account using equation 20.
Equation 20:
where the protein concentration is in mg/ml and k is an empirical coefficient equal to 0.009 ml/mg for spherical proteins. (Ref. 21 22 27 28) Sednterp will automatically perform this calculation if the user enters the concentration of protein in mg/ml. The user may also input other values of k by clicking on the s20w label on the sample form. Much larger values of k are expected for asymmetric proteins and the value of 0.009 should be used only if it is known that the protein is spherical or nearly so. There has not been a systematic study of the variation in k with axial ratio, and this might make a useful area for study.